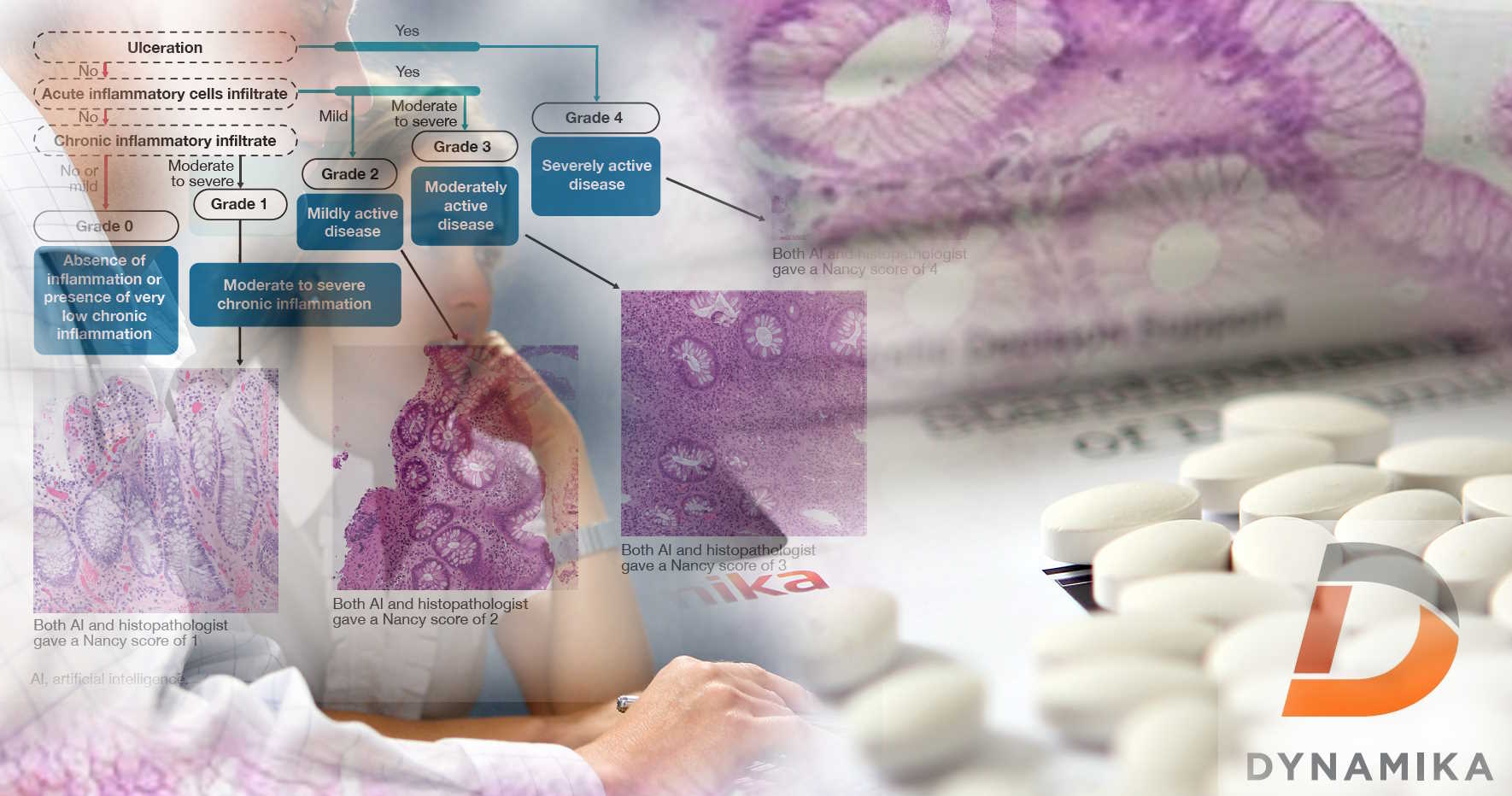
Join IAG’s Expert Team at EULAR 2023: Novel ideas for drug development
IAG is pleased to announce several publications at the upcoming EULAR 2023 conference. These publications represent IAG’s ongoing commitment to advancing the field of rheumatology through innovative research and collaboration. Join our team and collaborators who will be discussing the value of novel scoring system to improve clinical trials’ outcomes.
“Today, clinical trials require designs and endpoints which can give quick definitive answers about novel treatments in smaller patient cohorts and allow determining competitive edge of a novel drug candidate,” said Olga Kubassova, CEO and co-founder of IAG. “This is a great opportunity to showcase our research and support biotech and pharma collaborators.”
Below is a list of posters and presentations:
- MRI Digital Artery Volume Index (DAVIX©) as quantitative, continuous imaging outcome measure of vascular disease in systemic sclerosis – Poster: POS1336
- Efficacy of Apremilast on Whole-Body Inflammation Indices in Patients With Psoriatic Arthritis: Assessments by Whole-Body Magnetic Resonance Imaging in the Phase 4 MOSAIC Study – Publication: AB1535
- Apremilast Reduces Inflammation as Measured by MRI of the Hand in Patients With Psoriatic Arthritis: Primary Results from the Phase 4 MOSAIC Study – Poster: POS0226
- A Proof of Biological Concept Trial of CM101 to Target CCL24 in Systemic Sclerosis: a Biomarker Informed, Precision Medicine Approach – Publication: AB0808
- A Phase 2b double-blind placebo-controlled randomized clinical trial of SB-061, an aggrecan mimetic, in subjects with symptomatic knee osteoarthritis – Poster: POS0104
- Five-Year Follow-Up Of A 2-Year Mri Treat-To-Target Strategy On Radiographic Damage Progression In Rheumatoid Arthritis Patients In Clinical Remission – The Imagine-More Study – Poster: POS0052
- Intra-Articular Mm-Ii, A Novel Suspension Of Large Empty Multilamellar Liposomes, In Painful Knee Osteoarthritis: A 26 Week Phase 2b Randomized Trial – Poster: POS0106
- The Influence Of Baseline Demographics On Variability Of Oa Pain Assessed By Womac Pain Change From Baseline In Interventional Trials – Poster:POS1361
- Colchicine Twice Daily For Hand Osteoarthritis: Results From The Double-Blind, Randomised, Placebo-Controlled Color Trial – Presentation: OP0073
- 2023 Update Of Eular Recommendations For The Treatment Of Systemic Sclerosis – Presentation: OP0234
We will provide further updates on the specific details of these publications as they become available.
“It is important to us that our work is accessible to the broader scientific community,” said Prof. Mikael Boesen, chair of Scientific Advisory Board of IAG.
IAG looks forward to participating in EULAR 2023 and contributing to the ongoing advancement of the field of rheumatology.
Join IAG’s scientific team at EULAR 2023, May 30 – June 2 2023, Milan, Italy, at our poster presentations!
About IAG, Image Analysis Group:
IAG, Image Analysis Group is a unique clinical development partner to life sciences companies. We broadly leverage our proprietary image analysis methodologies, power of our cloud platform DYNAMIKA, years of experience in AI and Machine Learning as well as bespoke co-development business models to ensure higher probability for promising therapeutics to reach the patients. Our independent Bio-Partnering division fuses risk-sharing business models and agile culture to accelerate novel drug development. wp1.ia-grp.com
Learn more: wp1.ia-grp.com
Reach out: imaging.experts@ia-grp.com
Follow the Company: Linkedin